Gene therapy is being used to conduct breakthrough research for conditions such as hemophilia and rare inherited lysosomal storage disorders such as Fabry’s and Gaucher’s disease. While gene therapy as a technique is promising, it is still in its nascent stages and FDA guidance on analytical strategies for gene therapy is sparse. A key challenge is identifying those patients who are most likely to benefit, and therefore all stakeholders—developers, regulators, providers, patients, and payers—are seeking assurances that the therapy will work in the target population.
Immunogenicity plays an important role in the success or failure of a gene therapy as pre-existing immunity may limit the product’s therapeutic potential. Adeno-associated virus (AAV) vectors are the leading platform for transgene delivery, and as more than 50% of the general population has some degree of pre-existing immunity to serotypes of AAV, the assessment of specific anti-AAV antibodies is a significant consideration in gene therapy development. This holds true even if the AAV has been modified or if the strain used is not commonly seen in humans since there is significant cross-reactivity across serotypes.
Comparing Methods for Detecting Antibodies
The FDA Guidance, Human Gene Therapy for Rare Diseases, acknowledges the risk associated with pre-existing antibodies and recommends that developers “strongly consider contemporaneous development of a companion diagnostic to detect antibodies” to the gene therapy product [1]. The agency has also released draft guidance on Assay Development and Validation for Immunogenicity Testing of Therapeutic Protein Products, but has not provided specific guidance on how to select the appropriate immunogenicity assay.
The most common antibody detection methods are total antibody (TAb) and neutralizing antibody (NAb) assays (see Table 1).
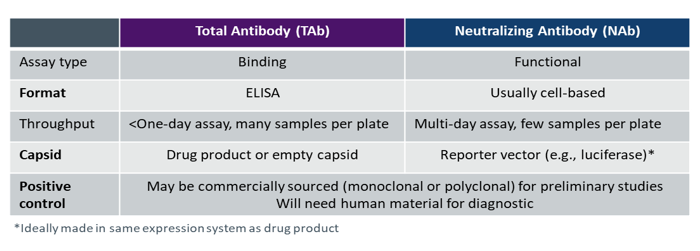 Table 1. Comparison of methods for detecting antibodies
Table 1. Comparison of methods for detecting antibodies
TAb immunogenicity tests are simpler to design, develop, and implement as they are standard enzyme-linked immunosorbent assays (ELISAs) for detecting binding. Multiple samples can be placed on a plate and the assay can be completed in hours using technology that is available in most labs. TAb assays are also easy to kit and ship, which streamlines clinical trial logistics.
NAb assays are functional, typically cell-based tests that require a reporter vector such as a luciferase construct. These assays are used to determine whether there are antibodies present in the sample that neutralize activity. They are, however, complex to develop and utilize in a regulatory setting as they must reflect the mechanism of action of the gene therapy product.
Selecting an Immunogenicity Assay
Deciding which type of immunogenicity assay to use can be challenging. This is especially true because while neutralizing antibodies are generally a subset of total antibodies, there are exceptions. Though this continues to be a subject of vigorous debate, here are some factors to consider when selecting an immunogenicity assay:
- Using a TAb assay would likely exclude more samples than a NAb, which could limit clinical utility,
- Age and previous treatments of the intended population may impact antibody levels,
- Existing animal or other preclinical data may provide insight on which assay is most relevant,
- If feasible, running both TAb and NAb assays in preclinical studies may help clarify which assay is more clinically relevant.
Banking extra serum or plasma samples in case additional assays need to be run in subsequent studies is generally a wise practice and ethically acceptable.
Correlating Immunogenicity with Efficacy
Regardless of the assay method, correlating pre-existing immunity with efficacy of a gene therapy is nuanced and determining what level or type of immunogenicity is clinically relevant is challenging, requiring clinical studies. To date, there is limited evidence that elevations in NAb to AAV are relevant to the efficacy of gene therapy, but the immunogenicity data being gathered now will inform the future of this space.
Developers are tasked with designing an assay and then correlating assay results with a clinical response. While preclinical studies can be used to determine a technical cut-off that can be used for further testing, clinical trials are necessary for identifying a clinical cut-off for pre-existing immunity that maximizes a gene therapy product’s therapeutic potential.
The ideal immunogenicity assay can correlate degree of immune response with efficacy and accurately determine which individuals are most likely to benefit from treatment. If immunogenicity impacts efficacy, it is likely that the assay selected in early-stage studies will need to be a companion diagnostic (CDx). With thoughtful, proactive planning, CDx development can occur in parallel with drug development—with a comprehensive plan for immunogenicity in the context of a clinical endpoint—streamlining the regulatory process and accelerating access to these life-changing therapies.
Reference
1. U.S. Food and Drug Administration. Human Gene Therapy for Rare Diseases: Guidance for Industry. Published January 2020. Available at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/human-gene-therapy-rare-diseases.